Guideline Digest
Bipolar Disorder: High and Low and What You Should Know
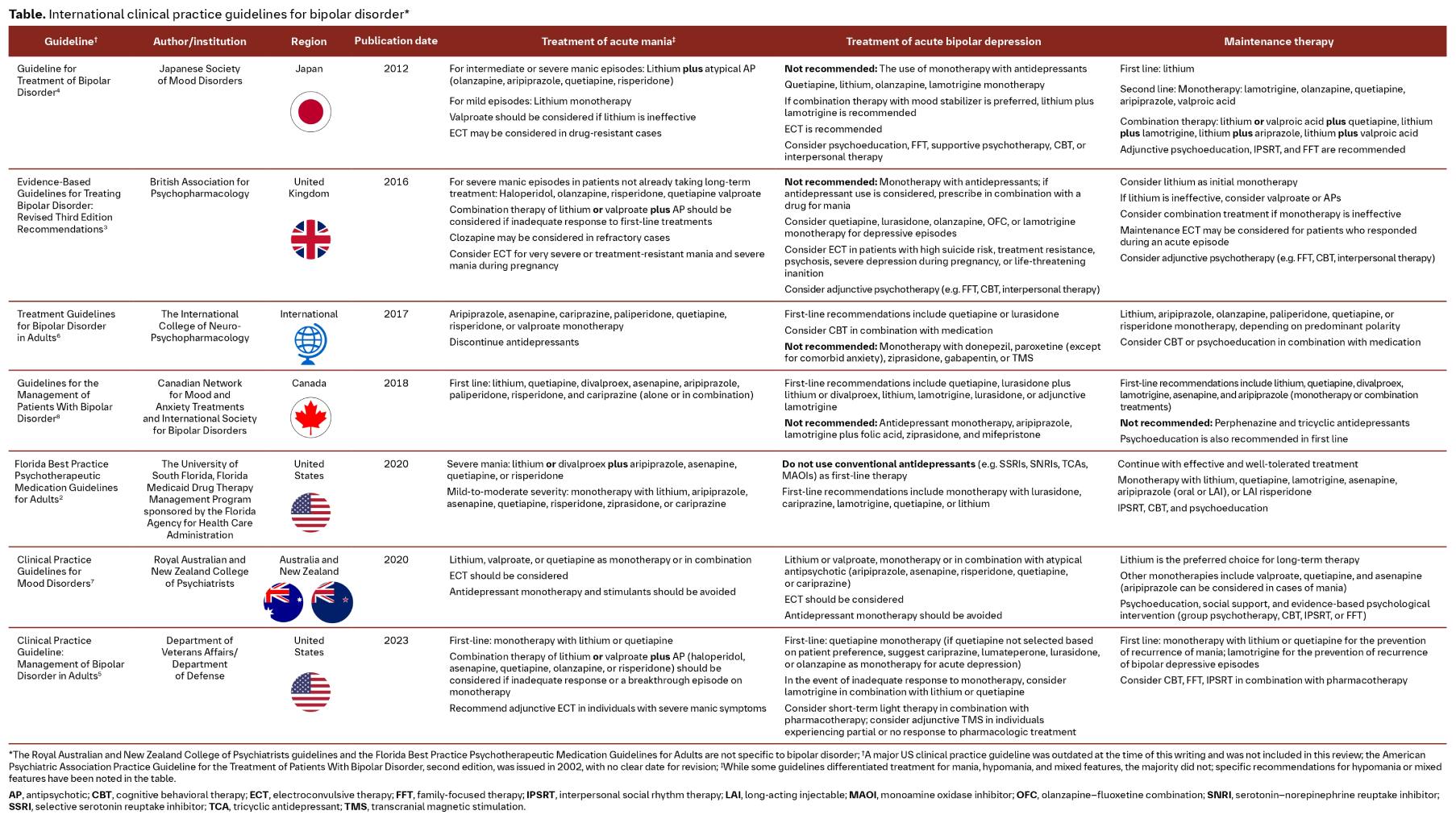
In this article series, we look at clinical guidelines from around the globe. For a feasible approach, we have chosen a sample of eight international guidelines for this review on the management of bipolar disorder (BD). The purpose is not to make an in-depth comparison of all existing guidelines, but to highlight the areas of greatest overlap and considerable differences.
In Summary
|
BD is a prevalent mental health condition characterized by recurrent shifts in mood that range from mania to severe depression.1 Psychotic symptoms such as delusions and hallucinations may also occur in ≤75% of manic episodes.1 In addition to the burdensome symptomatology, BD carries with it substantially higher rates of suicidality than the general population, high rates of coexisting psychiatric conditions that can increase the burden of illness and worsen prognosis, and high rates of comorbid chronic medical conditions such as metabolic syndrome and migraine.1 Together, these features compromise psychosocial functioning and make BD a leading cause of disability worldwide.1
Most patients with BD initially seek help from their primary care physician.1 Here we review eight clinical practice guidelines from around the globe and discuss current recommendations for the management of BD. A summary of the covered guidelines is provided in the Table.
Recommendations for the treatment of acute mania are generally consistent across clinical practice guidelines
Guidelines generally recommend treatment with a mood stabilizer and/or an antipsychotic as first-line options for adults with acute mania. Two of the guidelines reviewed differentiate between the treatment of mild, moderate, and severe manic symptoms,2,4 while the remaining guidelines broadly categorize treatment of acute mania. Combination therapy with lithium or valproate plus an antipsychotic is most often recommended for severe mania2,4 or an inadequate response to first-line monotherapy;3,5 whereas monotherapy with lithium, valproate, or an antipsychotic is often recommended for milder symptoms.2,4 Of the guidelines that do not differentiate between treatments based on the severity of acute manic symptoms, two recommend monotherapy with either a mood stabilizer or an antipsychotic,5,6 and two recommend monotherapy with either a mood stabilizer or an antipsychotic or combination therapy.7,8 Only the National Institute for Health and Care Excellence guidelines recommend only antipsychotics as first-line monotherapy for acute mania.9
Across guidelines, second-generation antipsychotics are usually preferred over first-generation antipsychotics, although haloperidol is an option in the British Association for Psychopharmacology, the US Department of Veterans Affairs/Department of Defense (VA/DoD), and the National Institute for Health and Care Excellence guidelines for the treatment of acute mania.3,5,9
Outside of pharmacotherapy, electroconvulsive therapy is another commonly recommended treatment for acute mania, particularly for severe or drug-resistant symptoms.3–5,7
Guideline consensus is that antidepressants should not be used for the treatment of bipolar depression
When symptomatic, individuals with BD spend 70%–80% of their time in a depressive episode.10 Consequently, the initial presentation of BD may resemble major depressive disorder, and therefore misdiagnosis is common.11 Unfortunately, commonly prescribed antidepressants may exacerbate BD by promoting mixed or manic symptoms or inducing mood instability or cycle acceleration.12 As a result, six of the eight guidelines reviewed recommend against the use of antidepressant monotherapy for the treatment of bipolar depression.2–4,7–9 Notably, the 2023 VA/DoD guidelines do not make a recommendation either for or against antidepressants, citing insufficient evidence.5
Figure. Summary of approved medications for bipolar depression and their approving agencies13–19
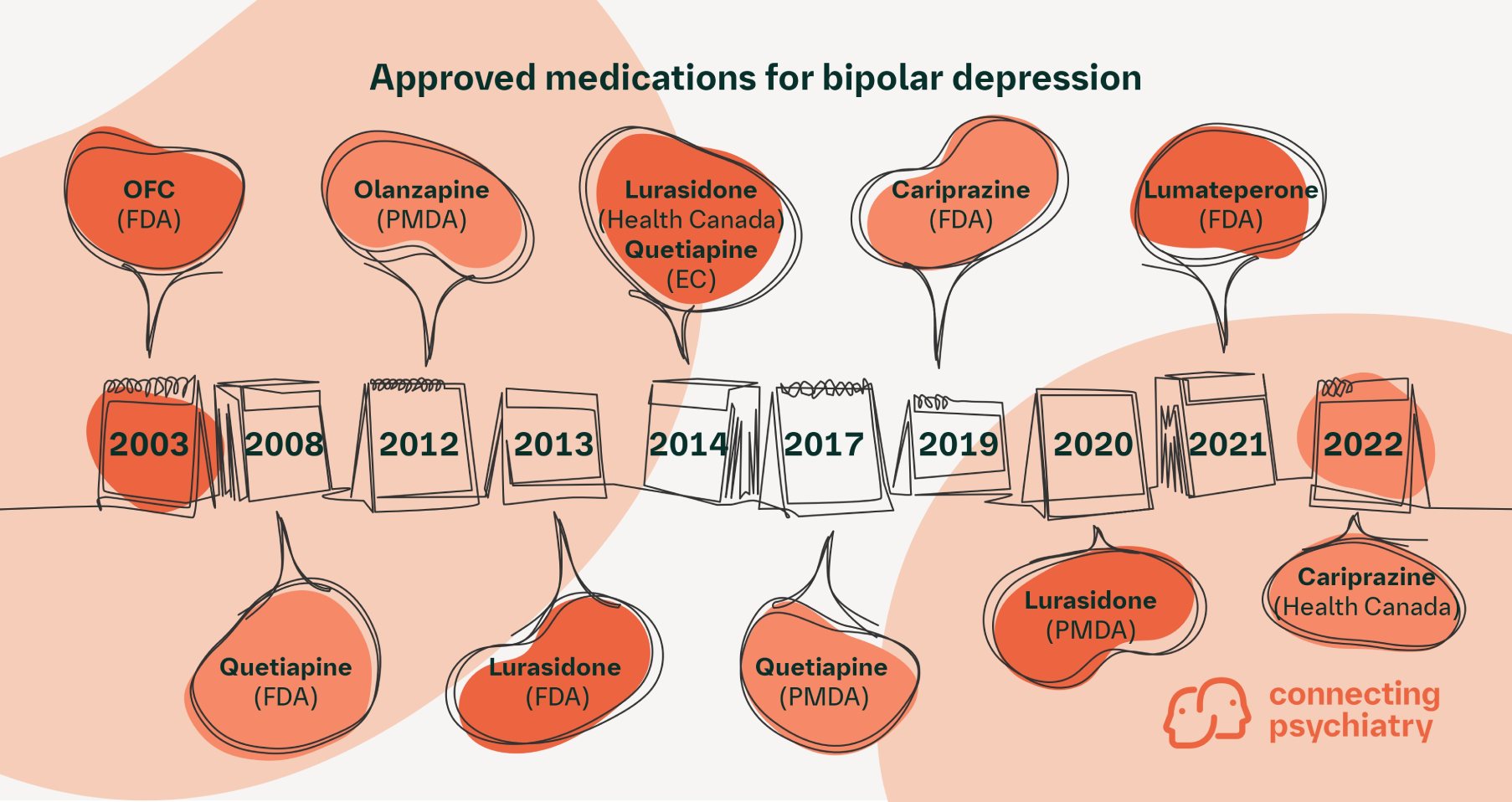
EC, European Commission (European Union); FDA, U.S. Food and Drug Administration (United States); OFC, olanzapine-fluoxetine combination; PMDA, Pharmaceuticals and Medical Devices Agency (Japan)
Several medications are currently indicated for bipolar depression in various regions of the world (Figure). In the United States, the Food and Drug Administration (FDA) has approved five products: four second-generation antipsychotics and one second-generation antipsychotic in combination with a selective serotonin reuptake inhibitor. Quetiapine is the only FDA-approved medication recommended by all eight guidelines reviewed,2–9 albeit as a second-line recommendation after mood-stablizing agents by the Royal Australian and New Zealand College of Psychiatrists guidelines. Lurasidone is also commonly recommended among the FDA-approved agents.2,3,6–8 Lithium2,4,5,7,8 and lamtotrigine2–5,7–9 have also been recommended by some guidelines, although they have not been approved for bipolar depression. The figure shows a timeline of approvals for medications for bipolar depression, including the regulatory body that granted the approval. It is worth referring to this timeline and noting the publication date of the guidelines discussed here (see Table); for example, the Japanese guidelines were published in 2012 prior to approvals, including those for lurasidone.
Electroconvulsive therapy and psychotherapy, including cognitive behavioral therapy, psychoeducation, family-focused therapy, and interpersonal therapy, are other recommended treatments for bipolar depression.2–4,6,7,9 Notably, the 2023 VA/DoD guidelines recommend transcranial magnetic stimulation in individuals who experience a partial response or no response to pharmacotherapy.5
The chronic and recurring nature of bipolar disorder makes maintenance treatment an important aspect of therapy
As lithium is one of the most effective medications for the prevention of both manic and depressive episodes of BD,1 it is no surprise that it is recommended as a first-line agent by all eight guidelines reviewed.2–9 In addition, psychotherapy is recommended by all guidelines reviewed as an adjunct to medication.2–6,8,9
Despite guideline-based care, many patients with BD continue to experience poor outcomes,12 representing an unmet need in the treatment landscape
BD has many recommended treatment options, and in general, the guidelines reviewed here allow for choice among first-line agents, acknowledging patient preference, severity of symptoms, and history of response to or tolerability of medications. However, a substantial number of patients do not respond well to currently available treatments and continue to have poor outcomes despite guideline-based care.12 For example, patients treated for bipolar depression still experience depressive symptoms approximately 19% of the time,13 and ≤40% of adults with BD in remission experience cognitive symptoms.20 Together, these residual symptoms contribute to substantial impacts on social and vocational functioning, poorer quality of life, and increased risk for suicide13 and represent an unmet need in the treatment landscape for BD.
Disclaimer: This document is for educational purposes and is not intended to replace approved clinical guidelines. Readers are advised to refer to their country- or region-specific guidelines when making clinical decisions.
Further reading
Carvalho AF, et al. Bipolar disorder. N Engl J Med 2020;383:58–66.
Mood fluctuations are common in daily life; however, when they become severe and persistent, they may be indicative of an underlying affective disorder such as bipolar disorder. This article presents a comprehensive review of bipolar disorder, including the epidemiology and burden, potential genetic and neurobiological causes, and management.Roosen L & Sienaert P. Evidence-based treatment strategies for rapid cycling bipolar disorder, a systematic review. J Affect Disord 2022;311:69–77.
Rapid cycling is a severe and disabling phase of bipolar disorder that often poses a major challenge to the clinician. This paper provides an overview of the evidence-based treatment options for rapid cycling.Papiol S, et al. Lithium response in bipolar disorder: Genetics, genomics, and beyond. Neurosci Lett 2022;785:136786.
Lithium is an effective mood stabilizer in bipolar disorder; however, the treatment response to lithium is highly variable, and only 20%–30% of individuals with BD are excellent responders. This article explores genetics, genomics, and other characteristics that may help us better understand the complex biological underpinnings of lithium treatment response.
BD, bipolar disorder; FDA, U.S. Food and Drug Administration; VA/DoD, US Department of Veterans Affairs/Department of Defense.
-
Carvalho AF, et al. N Engl J Med 2020;383:58–66.
-
University of South Florida, Florida Medicaid Drug Therapy Management Program sponsored by the Florida Agency for Health Care Administration. 2019–2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. 2020. Available at: https://floridabhcenter.org/wp-content/uploads/2021/04/2019-Psychotherapeutic-Medication-Guidelines-for-Adults-with-References_06-04-20.pdf. Last accessed: February 2024.
-
Goodwin GM, et al. J Psychopharmacol 2016;30:495–553.
-
Kanba S, et al. Psychiatry Clin Neurosci 2013;67:285–300.
-
Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Bipolar Disorder. 2023. Available at: https://www.healthquality.va.gov/guidelines/MH/bd/VA-DoD-CPG-BD-Full-CPGFinal508.pdf. Last accessed: February 2024.
-
Fountoulakis KN, et al. Int J Neuropsychopharmacol 2017;20:180–195.
-
Malhi GS, et al. Aust N Z J Psychiatry 2021;55:7–117.
-
Yatham LN, et al. Bipolar Disord 2018;20:97–170.
-
National Institute for Health and Care Excellence. Bipolar Disorder: Assessment and Management. 2014. Available at: https://www.nice.org.uk/guidance/cg185. Last accessed: February 2024.
-
Forte A, et al. J Affect Disord 2015;178:71–78.
-
Connecting Psychiatry. Major depressive disorder or bipolar disorder? A case of mistaken identity. Published May 2023. Available at: https://pro.boehringer-ingelheim.com/connecting-psychiatry/in-the-clinic/bipolar-or-mdd. Last accessed: February 2024.
-
Goes FS. BMJ 2023;381:e073591.
-
Levenberg K & Cordner ZA. Gen Psychiatr 2022;35:e100760.
-
European Medicines Agency. Questions and answers on Seroquel, Seroquel XR and associated names (quetiapine). Available at: https://www.ema.europa.eu/en/documents/referral/questions-answers-seroquel-seroquel-xr-associated-names-quetiapine_en.pdf. Last accessed: February 2024.
-
Cision. Health Canada approves Vraylar (cariprazine) for the treatment of bipolar I disorder and schizophrenia in adults. Available at: https://www.newswire.ca/news-releases/health-canada-approves-vraylar-r-cariprazine-for-the-treatment-of-bipolar-l-disorder-and-schizophrenia-in-adults-867345766.html#:~:text=MONTREAL%2C%20April%2027%2C%202022%20%2F,with%20bipolar%20l%20disorder%20in. Last accessed: February 2024.
-
Sumitomo Pharma. Press Release. Dainippon Sumitomo Pharma announces Health Canada approval of Latuda (lurasidone HCl) as monotherapy and adjunctive therapy in patients with bipolar depression. Available at: https://www.sumitomo-pharma.com/news/20140403.html. Last accessed: February 2024.
-
Pharmaceutical and Medical Devices Agency. New drugs approved in FY 2017. Available at: https://www.pmda.go.jp/files/000232769.pdf. Last accessed: February 2024.
-
Pharmaceutical and Medical Devices Agency. Review report. Available at: https://www.pmda.go.jp/files/000224954.pdf. Last accessed: February 2024.
-
Belmaker R. Am J Psychiatry 2014;171:131–133.
-
Tamura JK, et al. CNS Spectr 2021:1–22.
SC-US-76933
SC-CRP-15249
March 2024
Related content

Guideline Digest: Post-traumatic Stress Disorder
Sign up now for new features
The newsletter feature is currently available only to users in the United States
*Required field